



Every
Transplant
Matters
A briefing and recommendations for policymakers on improving post-transplant care
This report has been initiated, organised and funded by Takeda with insights from an expert working group.
C-ANPROM/EUC/MARI/0015 June 22
Copyright 2022 Takeda Pharmaceutical Company Limited. All rights reserved. Takeda and the Takeda logo are registered trademarks of Takeda Pharmaceutical Company limited





Every Transplant Matters
FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References




Contents
It is with great pleasure in my capacity as president of the European Society for Organ Transplantation to introduce the readership to this important document advocating for the improvement of post-transplant care.
Solid-organ transplantation is a set of therapeutic strategies that may grant a new life to many people affected by deadly diseases by involving broad and multidisciplinary expertise. Transplantation relies on the gift of an organ: the value of this gift is enhanced by the quality of care delivered after transplantation, leading to a longer and better life. Therefore, it is an imperative duty of all the stakeholders involved to foster clinical care, scientific research and healthcare organization to achieve long-term survival with good quality of life.
I spent over 20 years of my life in the care of post-transplant recipients, facing many diverse complications that these patients may suffer from. I have been feeling the challenges of managing patients with poor supporting scientific evidence, improving literacy in the communication with patients and families, caring for patients living far from the transplant centre, and of coping with limited resources for the care of complex diseases that are rare in the general population, but frequent in transplant recipients. Takeda has tackled these and other challenges in this document.
By using relevant case studies about the management of CMV infection as a model to describe the optimal process of care, a panel of 9 outstanding experts identified three major areas of improvement by policy changes: 1) patient engagement and empowerment; 2) enhancing posttransplant care delivery; 3) fostering research, innovation, and data sharing. To achieve these goals, this document endorses a decalogue of recommendations that are crucial to guide stakeholders in setting up actions to improve post-transplant care.
I believe that this approach sets a model for the industry to engage with the community effectively, with scientific societies and policymakers to change the perspective of transplantation from the success of a surgical procedure to a long-term benefit for the lives of patients supported by continuous improvement and innovative care.
Foreword

3
It is with great pleasure in my capacity as president of the European Society for Organ Transplantation to introduce the readership to this important document advocating for the improvement of post-transplant care.
Solid-organ transplantation is a set of therapeutic strategies that may grant a new life to many people affected by deadly diseases by involving broad and multidisciplinary expertise. Transplantation relies on the gift of an organ: the value of this gift is enhanced by the quality of care delivered after transplantation, leading to a longer and better life. Therefore, it is an imperative duty of all the stakeholders involved to foster clinical care, scientific research and healthcare organization to achieve long-term survival with good quality of life.
I spent over 20 years of my life in the care of post-transplant recipients, facing many diverse complications that these patients may suffer from. I have been feeling the challenges of managing patients with poor supporting scientific evidence, improving literacy in the communication with patients and families, caring for patients living far from the transplant centre, and of coping with limited resources for the care of complex diseases that are rare in the general population, but frequent in transplant recipients. Takeda has tackled these and other challenges in this document.
By using relevant case studies about the management of CMV infection as a model to describe the optimal process of care, a panel of 9 outstanding experts identified three major areas of improvement by policy changes: 1) patient engagement and empowerment; 2) enhancing posttransplant care delivery; 3) fostering research, innovation, and data sharing. To achieve these goals, this document endorses a decalogue of recommendations that are crucial to guide stakeholders in setting up actions to improve post-transplant care.
I believe that this approach sets a model for the industry to engage with the community effectively, with scientific societies and policymakers to change the perspective of transplantation from the success of a surgical procedure to a long-term benefit for the lives of patients supported by continuous improvement and innovative care.
Foreword

Dr Luciano Potena
Director Heart Failure and Transplant Unit
IRCCS Azienda Ospedaliero-Universitaria di Bologna
President of the European Society for Organ Transplantation
Every transplant offers a patient the opportunity of more years lived in better health. For every patient who receives a transplant, there are many more who are waiting in hope.
In the decades since the first organ and bone marrow transplants, scientists and doctors have improved our understanding of transplant techniques and post-transplant care, and many transplant patients can now live to a near-normal life expectancy. Yet there is still more we can do to give every patient the best possible chance of a successful transplant and a longer, healthy life.
Transplant care
Overview
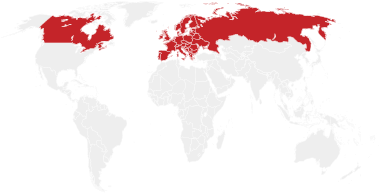
Every year, more than 70,000 people across Europe and Canada will have a life-changing transplant.1,2
Life-changing transplant
4
Introduction
Takeda is a patient-focused, values-based, R&D-driven global biopharmaceutical company committed to bringing better health and a brighter future to people worldwide. At Takeda, everything starts by asking: How can we do more for patients?
For every transplant to carry the best possible chance of success, transplant teams must be resourced and supported to deliver great care, from preparation, to transplant, and follow-up care. As part of this, efforts are made to prevent and treat common viral infections to which transplant patients are particularly vulnerable, such as cytomegalovirus (CMV).
Transplant teams work extremely hard to prevent or proactively manage CMV and other viral infections when they occur but, for a small number of patients, these infections can still cause significant problems after their transplant.5
Takeda sought to understand how a supportive policy environment could facilitate national transplant services and patient organisations to enhance transplant care for every patient, with a particular focus on tackling post-transplant infections. Policymakers have crucial roles to play in setting national priorities, encouraging investment into health and care services, and sharing of best practice both within and between different countries.
Takeda therefore sets out to analyse the policy landscape for transplant services in six countries – Canada, England, France, Germany, Italy and Spain – aiming to identify commonalities and points of divergence, best practices, and to suggest potential policy solutions where possible.
This briefing sets out key themes emerging from this landscape analysis and subsequent discussions with expert clinicians in solid organ and stem cell transplantation and patient representatives from across Europe and Canada. It also sets out recommendations for how policymakers can support transplant services to effectively tackle post-transplant infections.

Joe Brice
Head of Franchise Public Affairs & Policy, Europe & Canada
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

Every transplant offers a patient the opportunity of more years lived in better health. For every patient who receives a transplant, there are many more who are waiting in hope.
In the decades since the first organ and bone marrow transplants, scientists and doctors have improved our understanding of transplant techniques and post-transplant care, and many transplant patients can now live to a near-normal life expectancy. Yet there is still more we can do to give every patient the best possible chance of a successful transplant and a longer, healthy life.
Improving post-transplant care
Transplant care
Overview

Every year, more than 70,000 people across Europe and Canada will have a life-changing transplant.1,2
Life-changing transplant
5
Every year, over 30,000 more patients will have a different kind of transplant – a stem cell or bone marrow transplant – to treat conditions such as leukaemia or lymphoma.

Introduction
Takeda is a patient-focused, values-based, R&D-driven global biopharmaceutical company committed to bringing better health and a brighter future to people worldwide. At Takeda, everything starts by asking: How can we do more for patients?
For every transplant to carry the best possible chance of success, transplant teams must be resourced and supported to deliver great care, from preparation, to transplant, and follow-up care. As part of this, efforts are made to prevent and treat common viral infections to which transplant patients are particularly vulnerable, such as cytomegalovirus (CMV).
Transplant teams work extremely hard to prevent or proactively manage CMV and other viral infections when they occur but, for a small number of patients, these infections can still cause significant problems after their transplant.5
Takeda sought to understand how a supportive policy environment could facilitate national transplant services and patient organisations to enhance transplant care for every patient, with a particular focus on tackling post-transplant infections. Policymakers have crucial roles to play in setting national priorities, encouraging investment into health and care services, and sharing of best practice both within and between different countries.
Takeda therefore sets out to analyse the policy landscape for transplant services in six countries – Canada, England, France, Germany, Italy and Spain – aiming to identify commonalities and points of divergence, best practices, and to suggest potential policy solutions where possible.
This briefing sets out key themes emerging from this landscape analysis and subsequent discussions with expert clinicians in solid organ and stem cell transplantation and patient representatives from across Europe and Canada. It also sets out recommendations for how policymakers can support transplant services to effectively tackle post-transplant infections.
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References


6
Every Transplant Matters


Nearly 39,000 people annually will receive the gift of a kidney, heart, lung, liver, pancreas, or small bowel transplant. Most of these will be donated organs and tissue from people who have died. In some cases, living donors (often family members) can donate a kidney or part of their liver.












Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

7
Improving post-transplant care
Acknowledging the experts
We extend our grateful thanks to the experts who contributed their unique insights to this project:



Marion
Patient Representative, Renaloo, France
Dr Mélanie Dieudé
Executive Director, The Canadian Donation and Transplantation Research Program (CDTRP), Canada
Daniel Gallego
President, European Kidney Patients Federation (EKPF) and President, Asociación para la Lucha Contra las Enfermedades del Riñón (ALCER, Association for the Fight against Kidney Diseases), Spain



Dr Corrado Girmenia
Haematology Institute, Azienda Ospedaliero – Universitaria Policlinico Umberto 1, Rome, Italy
Professor Nassim Kamar
Head of the Department of Nephrology and Organ Transplantation, Toulouse University Hospital, France
Professor Umberto Maggiore
Head of Kidney-Pancreas Transplantation Unit, University of Parma, Italy



Professor Antonio Pagliuca
Chief Medical and Scientific Adviser to Anthony Nolan and Trustee of Leukaemia UK, United Kingdom
Dr Michael Perch
Medical Director of the National Danish Lung Transplant Program; Section Chief, Section for Lung Transplantation, Heartcentre, Rigshospitalet,University Hospital of Copenhagen, Denmark
Dr José Luis Piñana
Haematology division, Hospital Clinico Universitario of Valencia, Valencia, and Infectious and Non-Infectious Complications Working Group Coordinator, Grupo Español de Trasplante Hematopoyético y Terapia Celular (GETH, Spanish Hematopoietic Stem Cell Transplantation and Cell Therapy Group), Spain
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

8
Executive summary
A transplant is a precious opportunity for every patient who needs one, offering them the best available replacement therapy and improving their quality and length of life. It is a vital investment by the healthcare system in the patient’s future life and health, and can also save healthcare resources by removing the need for other treatments. We need to do what we can to give every patient the best possible chance of their transplant being successful and them living a longer, healthy life.
The immunosuppressant medicines that patients need to take for life to stop their transplant being rejected also reduce the body’s resistance to infections. Common infections like CMV, which may not cause any symptoms for healthy adults, can (re)activate, and may become a serious problem for some transplant patients.
For this reason, treatment teams are especially vigilant for post-transplant infections. Advances in preventive or prophylactic therapies and antiviral treatments, coupled with international best practice clinical guidelines for both solid organ and stem cell transplants, mean that many post-transplant infections can be successfully managed. However, for some patients, a CMV infection can still have serious consequences.
Policymakers have crucial roles to play in supporting high quality patient-centred transplant services, including through setting national priorities, encouraging investment into health and care services, and sharing of best practice both within and between different countries. Policymakers can also encourage scrutiny of national services, to identify variations in approach and performance.
Takeda commissioned an analysis of transplant services and post-transplant care in six countries, validated with international experts. From this, we have identified three themes under which policy changes could support optimisation of patient journeys and outcomes: patient engagement and empowerment, enhancing post-transplant care delivery; and fostering research, innovation, and data sharing.
We recognise that national landscapes and approaches vary, and there is no one-size-fits-all approach. The policy recommendations included here are intended as a starting point for further discussions with national policymakers, system and clinical leaders, and patient organisations.
Takeda is keen to partner with all interested parties to explore these findings and how we can support delivery of still better outcomes for people who have a transplant.
Every Transplant Matters
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

Policy recommendations
Executive summary
A transplant is a precious opportunity for every patient who needs one, offering them the best available replacement therapy and improving their quality and length of life. It is a vital investment by the healthcare system in the patient’s future life and health, and can also save healthcare resources by removing the need for other treatments. We need to do what we can to give every patient the best possible chance of their transplant being successful and them living a longer, healthy life.
The immunosuppressant medicines that patients need to take for life to stop their transplant being rejected also reduce the body’s resistance to infections. Common infections like CMV, which may not cause any symptoms for healthy adults, can (re)activate, and may become a serious problem for some transplant patients.
For this reason, treatment teams are especially vigilant for post-transplant infections. Advances in preventive or prophylactic therapies and antiviral treatments, coupled with international best practice clinical guidelines for both solid organ and stem cell transplants, mean that many post-transplant infections can be successfully managed. However, for some patients, a CMV infection can still have serious consequences.
Policymakers have crucial roles to play in supporting high quality patient-centred transplant services, including through setting national priorities, encouraging investment into health and care services, and sharing of best practice both within and between different countries. Policymakers can also encourage scrutiny of national services, to identify variations in approach and performance.
Takeda commissioned an analysis of transplant services and post-transplant care in six countries, validated with international experts. From this, we have identified three themes under which policy changes could support optimisation of patient journeys and outcomes: patient engagement and empowerment, enhancing post-transplant care delivery; and fostering research, innovation, and data sharing.
We recognise that national landscapes and approaches vary, and there is no one-size-fits-all approach. The policy recommendations included here are intended as a starting point for further discussions with national policymakers, system and clinical leaders, and patient organisations.
Takeda is keen to partner with all interested parties to explore these findings and how we can support delivery of still better outcomes for people who have a transplant.
9
Improving post-transplant care
Recommendation 1
Commission research at national level to understand any variations in practice between different centres and why some transplant centres may choose to diverge in practice from international clinical guidance
Recommendation 4
Audit patients’ experience of transplant care, from pre-transplant planning to follow-up care, to identify variations, challenges and best practice, and to inform strategic planning at a national and local level
Recommendation 8
Stimulate collaboration and research into areas of high unmet need, including supporting European transplant registries and national transplant societies to collect and share evidence and data on key topics (e.g. optimal management of resistant/refractory CMV infections)











Recommendation 7
Explore and harness the potential of e-health and virtual consultations to better support transplant patients and their families closer to home
Recommendation 10
Enable more flexible value assessment models to assess innovations that address high unmet need in small patient populations or where real-world evidence is needed, incorporating patient voices and perspectives as a direct part of the assessment
Recommendation 3
Work with patient groups to translate international guidelines into patient-friendly language, so patients know what they should be expecting from their care
Recommendation 6
Resource local testing networks to enable patients to have follow-up transplant care closer to home, including monitoring for infections





















Recommendation 2
Ensure patients are given high quality, balanced information on the risk of post-transplant infections, as part of a comprehensive education package that recognises the complexity of patients’ lives post-transplant
Recommendation 5
Ensure transplant follow-up services are well-organised and well-funded with the multidisciplinary capacity and capabilities needed to support holistic patient needs
Recommendation 9
Work with national transplant societies, transplant centres and registries to better understand the incidence and burden of post-transplant infections, including CMV




Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References
















10
Transplants:
A life-saving opportunity
Every year, thousands of people across Europe and Canada have a transplant.6–8 For many, it will be a life-saving intervention, allowing them to live a more normal life for longer.
A transplant is a precious investment for every patient. It can also be a cost-effective intervention, saving the healthcare system resources by removing the need for other treatments.
Every Transplant Matters
In 2019/20, more than 3,000 people in Canada9 and 36,000 people in the EU had an organ transplant.10
The EU countries with most organ transplants were France (4,429), Spain (4,427), Germany (3,518) and Italy (3,503).11


















In 2019, around 1,400, people in Canada had an allogenic stem cell transplant,12 while in 2019, 19,798 people in the EU had an allogeneic stem cell transplant.13
The European countries with the highest rates of allogenic transplants were Denmark, Germany, Italy, Lithuania, Sweden (all at > 300 per 10 million population).14









Daniel’s video

Every Transplant Matters
Solid organ transplants
Canada (2019)
Europe (2020)
3,014



















Austria
672

Republic of Cyprus
13

Finland
408

Hungary
320

Lithuania
107

Poland
1,242

Slovenia
114

Belgium
756

Czech Republic
554

France
4,429

Ireland
190

Luxembourg
–

Portugal
680

Croatia
219

Estonia
65

Greece
219

Latvia
45

Netherlands
1,152

Slovakia
117

Sweden
719

UK
3,801

Bulgaria
16

Denmark
413

Germany
3,518

Italy
3,503

Malta
6

Romania
241

Spain
4,427



Every Transplant Matters
Stem cell transplants
Canada (2019)
Europe (2019)
2,706

Austria
566

Republic of Cyprus
9

Finland
207

Hungary
450

Lithuania
243

Poland
1,335

Slovenia
131

Belgium
1,101

Czech Republic
742

France
5,428

Ireland
297

Luxembourg
–

Portugal (2015)
539

Croatia
289

Estonia
85

Greece
–

Latvia
–

Netherlands (2017)
1,400

Slovakia
245

Sweden
908

UK (2018)
3,650

Bulgaria
160

Denmark
–

Germany
8,022

Italy
5,883

Malta
–

Romania
308

Spain
3,444











Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References
















11
Transplants:
A life-saving opportunity
Every year, thousands of people across Europe and Canada have a transplant.6–8 For many, it will be a life-saving intervention, allowing them to live a more normal life for longer.
A transplant is a precious investment for every patient. It can also be a cost-effective intervention, saving the healthcare system resources by removing the need for other treatments.
I was CMV negative before I had my kidney
transplant in November 2011. I got the CMV diagnosis
quite soon after the transplant when the medical team
explained my medication. It was presented very lightly, as a
normal thing related to transplantation. Later the nephrologist told
me that the team accepted the CMV positive organ from a (deceased)
donor because the overall match with the deceased donor was good.
I had never heard or thought of the transfer of a virus via a transplant. I did not have much knowledge of viruses and certainly not about CMV (I think everyone’s knowledge of viruses has evolved a bit since the COVID virus).
I could not find much information on the internet – it was 2012 and information on the internet was not yet as it is now – except that it is a virus that most adults have without being ill.
I was so happy with this new life. I had high confidence in the medical team, so I was not too worried. I had oral medication for 3 months and monitoring of my blood. I did not have signs of an infection, but apparently it was not ok yet and I was still infected with CMV.
I had further home treatment for 1 or 2 weeks by a daily intravenous infusion, which was not effective. I was then hospitalized for 1 or 2 weeks with another treatment and detailed monitoring, after which the doctors said it was ok and I was no longer infected with CMV and I never heard or spoke about CMV anymore.
Looking back now, I would like to understand more about what my medical team was monitoring. Was it the virus level in my blood? Was the virus resistant? I would also like to know the side-effects of the treatments, since this was not communicated to me. Ten years on from my transplant I still feel tired and need to sleep 11/24 hours, and doctors do not have a cause or solution.
I might have appreciated more information when I got the treatments, though it might have been lost in all the other information I had. It would have needed to be given carefully though, or I would have been stressed and dispirited for the total transplantation, and that is exactly what the doctors tried to prevent.
Patient story:
Marion’s story
Daniel’s video

Every Transplant Matters
Solid organ transplants


Canada (2019)
Europe (2020)
3,014



















Austria
672

Republic of Cyprus
13

Finland
408

Hungary
320

Lithuania
107

Poland
1,242

Slovenia
114

Belgium
756

Czech Republic
554

France
4,429

Ireland
190

Luxembourg
–

Portugal
680

Croatia
219

Estonia
65

Greece
219

Latvia
45

Netherlands
1,152

Slovakia
117

Sweden
719

UK
3,801

Bulgaria
16

Denmark
413

Germany
3,518

Italy
3,503

Malta
6

Romania
241

Spain
4,427



Every Transplant Matters
Stem cell transplants


Canada (2019)
Europe (2019)
2,706

Austria
566

Republic of Cyprus
9

Finland
207

Hungary
450

Lithuania
243

Poland
1,335

Slovenia
131

Belgium
1,101

Czech Republic
742

France
5,428

Ireland
297

Luxembourg
–

Portugal (2015)
539

Croatia
289

Estonia
85

Greece
–

Latvia
–

Netherlands (2017)
1,400

Slovakia
245

Sweden
908

UK (2018)
3,650

Bulgaria
160

Denmark
–

Germany
8,022

Italy
5,883

Malta
–

Romania
308

Spain
3,444











Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

12
Post-transplant infections:
the impact of CMV
After a transplant, patients will need to take lifelong immunosuppressant (anti-rejection) medicines to stop their body from rejecting the transplant. However, because immunosuppressants work by making the immune system less efficient, they also reduce the body’s resistance to infections.
Every Transplant Matters



Common infections, which may not cause any symptoms for healthy adults, can become a problem for transplant patients. For this reason, treatment teams are especially vigilant for infections, and may prescribe both preventive treatments to reduce risk, and therapies to manage infections where they occur (discussed in more detail in the next section).
Cytomegalovirus (CMV) is one of the viruses that can often affect patients after a transplant.16 CMV is related to the herpes virus that causes cold sores and chickenpox. Between 60−100% of adults will contract CMV at some point in their lives.17 Many people will not even realise they have it, while others may have cold-like symptoms such as sore throat, swollen lymph nodes, fatigue or fever. Once you have the virus it stays in your body for life.
Between 60−100% of adults will contract CMV at some point in their lives.17
60–100%
In most cases of post-transplant CMV infection, the dormant virus reactivates in the patient’s system while their immune system is suppressed by their post-transplant medications. It is also possible for CMV to be transferred from a positive donor to a negative patient through the donated organ or stem cells; careful pre-testing occurs to prevent this from happening wherever possible. Certain types of transplants carry a higher risk of CMV. Estimates suggest that around 16–56% of solid organ transplant patients.
Advances in preventive or prophylactic therapies and antiviral treatments mean that CMV can often be successfully managed but, for some patients, CMV can still have serious consequences. Transplant patients with CMV are 2–9 times more likely to have poor transplant function than those without CMV, affecting their physical
30–70% of stem cell transplant patients will experience an active CMV infection.18,19
30–70%





Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References


13
Post-transplant infections:
the impact of CMV
After a transplant, patients will need to take lifelong immunosuppressant (anti-rejection) medicines to stop their body from rejecting the transplant. However, because immunosuppressants work by making the immune system less efficient, they also reduce the body’s resistance to infections.
and emotional health and their overall quality of life.21,22 In the most severe cases, CMV can result in the transplanted organ being rejected, disease relapsing or even fatality.23,24 This carries a mental and physical burden for the patient but also an economic burden for the healthcare system; CMV infection post-transplant is associated with a significant increase in healthcare costs, both in solid organ25 and stem cell transplants.26
Before their transplant, the transplant team should discuss with the patient the risk of different infections. In the case of CMV, they should also explain the need for tests to see if the patient already has the virus and, if so, how this will affect their treatment plan; for example, if pre-emptive therapy is being given, the patient may need weekly viral monitoring tests to check whether the CMV has reactivated as there may not be obvious physical signs or symptoms.
Improving post-transplant care
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

14
Best practice in prevention and management of CMV infections
Experts have published international clinical guidelines to support transplant teams in preventing and managing CMV, with separate guidelines for solid organ transplants27 and stem cell transplants.28
Each guideline covers diagnostics, preventative strategies such as prophylaxis and pre-emptive treatment, and management of CMV disease.
Every Transplant Matters
Solid organ transplantation


Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, 2018

Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

15
Best practice in prevention and management of CMV infections
Experts have published international clinical guidelines to support transplant teams in preventing and managing CMV, with separate guidelines for solid organ transplants27 and stem cell transplants.28
Each guideline covers diagnostics, preventative strategies such as prophylaxis and pre-emptive treatment, and management of CMV disease.
Improving post-transplant care
Solid organ transplantation


Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, 2018

More research is needed to understand the reasons for this divergence from the guidelines (for example clinical knowledge and/or belief, conflict with national guidelines, access to diagnostics, cost implications or other factors).
their donor had CMV it may reactivate. The risk of this happening is not only affected by whether the donor or recipient had CMV before the transplant, but also by the type of solid organ transplant.33
The guidelines therefore make recommendations for either universal prophylaxis or pre-emptive therapies for prevention of CMV disease, with tailored recommendations on treatment approach and duration based on the type of organ transplant, the donor / recipient CMV serostatus, and therefore the risk level. They also advise on the preferred type and frequency for monitoring patients to detect CMV disease and assess the effectiveness of pre-emptive treatments.
The guidelines make additional recommendations on how to treat, manage and monitor CMV disease, should it arise, proposing an algorithm to support decision-making if the clinician suspects that the infection is becoming resistant to treatment. During the treatment phase, patients may need to be treated in hospital with intravenous therapies, being closely monitored to assess their response to treatment, to guide dosage adjustments, and to check for antiviral resistance.
There is known to be variation in uptake and use of the guidelines. A 2019 survey of transplant centres by the European Society on Organ Transplantation found differences in:34
The diagnostic tools that centres were using
The thresholds at which they were initiating pre-emptive therapy
Which patients were being given prophylaxis, for how long, and how they were monitored
The approach centres were taking to managing resistant CMV
International consensus guidelines for solid organ transplants were first published in 2010, and updated in 2013 and 2018.
The 2018 guidelines emphasize the importance of pre-transplant testing for CMV to determine whether the donor or recipient (D/R) have already been infected with CMV. The status of both donor and recipient are important predictors of the risk of CMV after transplant and guide clinical decisions on the best strategy to take to manage this. The guidelines note that D/R serostatus can strongly affect the outcome of the transplant.
Repeat testing at the time of transplant is recommended if the pre-transplantation testing was negative, so the transplant team have the most up to date information. The guidelines recommend using specific serological tests with high specificity and high sensitivity, since not all tests are equivalent.
Ideally, a CMV-negative donor will be chosen for a negative patient and a CMV-positive donor for a positive patient. However, in practice, this is not always possible. The results of the blood tests will help the transplant team determine the level of potential risk to the patient and decide whether they should have antiviral prophylaxis or pre-emptive treatment.
Before the transplant surgery, the patient will have high dose immunosuppressant therapy to prevent their body’s immune system from rejecting the organ or stem cells. However, this means that the patient is more susceptible to infection; if they or
The impact of donor / recipient CMV status on risk category32
* D+/R+ generally at higher risk than D-/R+
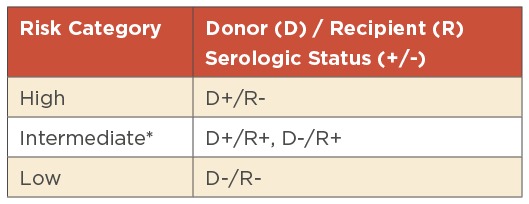
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

16
The European Society for Organ Transplantation (ESOT) sent out a survey to 8,000 contacts worldwide, seeking to understand the management of CMV and to evaluate whether international guidelines (Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation) were being followed.
Between July and October 2019, ESOT analysed 224 responses from clinicians working in adult and paediatric solid organ transplantation centres across the world (85% based in Europe).
The survey found variations in approaches to CMV management and divergence with the international guidelines in the following areas:
Every Transplant Matters
Case study:
CMV management practices across Europe: results of an ESOT survey
|
Area |
International guideline recommendation |
ESOT survey findings |
|
Diagnosing CMV |
Using whole blood or plasma CMV DNA test |
Many centres are testing using whole blood CMV DNA, but there are still a large number that use plasma CMV testing |
|
Initiating pre-emptive or preventative therapies |
Preventative therapies are given to CMV patients to help manage risk |
Centres are using pre-emptive therapies but there is large variation in the threshold for the level of CMV-DNA seen at which centres are initiating pre-emptive therapies |
|
Prophylaxis and monitoring |
Prophylaxis is favoured over the use of pre-emptive therapies in the highest risk recipients (eg D+/R-) Hybrid strategy (monitoring after prophylaxis is not recommended) |
For D+/R- patients: 90% of centres report using prophylaxis There is variation between centres in the type of prophylaxis given and its duration within transplant types For R+ patients: 47% of centres reported using prophylaxis, and 48% reported using pre-emptive therapy There was variation between centres in the duration of prophylaxis given within transplant types Monitoring approaches also varies with some centres monitoring once a week and others ranging from three to 12 months |
Stem cell transplantation


Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7), 2019

Expert recommendations were made by a working group within the 2017 European Conference on Infections in Leukaemia (ECIL 7) following a comprehensive literature review. These were presented and discussed in a plenary session of the ECIL 7, made available for open consultation from October 2017 to March 2018, and published in 2019.35
The guidelines highlight that CMV serological status of patients and donors strongly influences the outcome of HSCT, with CMV positive patients having poorer outcomes than CMV negative patients.36,37 They therefore recommend pre-transplant CMV testing of both patient and donor close to the time of the stem cell transplant.
Immunosuppressant medicines are not needed by patients who have an autologous transplant (using their own stem cells). However, patients who have an allogeneic transplant will take immunosuppressants to prevent graft versus host disease (GvHD), which happens when the new donor cells view the recipient’s body as foreign and attack the body. Studies have shown that GvHD and its treatment can induce CMV replication, and the reciprocal finding that patients are at significantly increased risk of developing acute GvHD during CMV replication.38
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

The European Society for Organ Transplantation (ESOT) sent out a survey to 8,000 contacts worldwide, seeking to understand the management of CMV and to evaluate whether international guidelines (Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation) were being followed.
Between July and October 2019, ESOT analysed 224 responses from clinicians working in adult and paediatric solid organ transplantation centres across the world (85% based in Europe).
The survey found variations in approaches to CMV management and divergence with the international guidelines in the following areas:
17
Improving post-transplant care
Case study:
CMV management practices across Europe: results of an ESOT survey
|
Area |
International guideline recommendation |
ESOT survey findings |
|
Diagnosing CMV |
Using whole blood or plasma CMV DNA test |
Many centres are testing using whole blood CMV DNA, but there are still a large number that use plasma CMV testing |
|
Initiating pre-emptive or preventative therapies |
Preventative therapies are given to CMV patients to help manage risk |
Centres are using pre-emptive therapies but there is large variation in the threshold for the level of CMV-DNA seen at which centres are initiating pre-emptive therapies |
|
Prophylaxis and monitoring |
Prophylaxis is favoured over the use of pre-emptive therapies in the highest risk recipients (eg D+/R-) Hybrid strategy (monitoring after prophylaxis is not recommended) |
For D+/R- patients: 90% of centres report using prophylaxis There is variation between centres in the type of prophylaxis given and its duration within transplant types For R+ patients: 47% of centres reported using prophylaxis, and 48% reported using pre-emptive therapy There was variation between centres in the duration of prophylaxis given within transplant types Monitoring approaches also varies with some centres monitoring once a week and others ranging from three to 12 months |
Stem cell transplantation


Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7), 2019

Expert recommendations were made by a working group within the 2017 European Conference on Infections in Leukaemia (ECIL 7) following a comprehensive literature review. These were presented and discussed in a plenary session of the ECIL 7, made available for open consultation from October 2017 to March 2018, and published in 2019.35
The guidelines highlight that CMV serological status of patients and donors strongly influences the outcome of HSCT, with CMV positive patients having poorer outcomes than CMV negative patients.36,37 They therefore recommend pre-transplant CMV testing of both patient and donor close to the time of the stem cell transplant.
Immunosuppressant medicines are not needed by patients who have an autologous transplant (using their own stem cells). However, patients who have an allogeneic transplant will take immunosuppressants to prevent graft versus host disease (GvHD), which happens when the new donor cells view the recipient’s body as foreign and attack the body. Studies have shown that GvHD and its treatment can induce CMV replication, and the reciprocal finding that patients are at significantly increased risk of developing acute GvHD during CMV replication.38
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

Every Transplant Matters
The guidelines also make recommendations around choice of donor based on CMV status:
The guidelines propose using specific tests for monitoring of CMV in plasma and whole blood, recommending weekly testing for the first 100 days and longer monitoring for patients in specific groups. Sensitive testing techniques allows intervention before development of CMV disease, and the guidelines recommend pre-emptive therapy, either as a stand-alone strategy or combined with antiviral prophylaxis.
The guidelines make additional recommendations for treatment of CMV disease with antiviral therapies. They note that resistance to antivirals is infrequent, varying between 0% and 10% of different patient populations,39 but that the treatment of resistant or refractory CMV infection and disease remains a major therapeutic challenge.
CMV negative donor to a CMV negative patient
A CMV positive donor for a CMV positive patient, where the donor is unrelated
Either a CMV positive or negative donor for a CMV positive recipient, where the donor is related
Weekly testing for the first 100 days
100














18
More research is needed to understand whether variation is seen in the approach taken between centres providing stem cell transplants, whether there is divergence from international guidelines, and the reasons for this if so.
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

19
Improving post-transplant care
The policy landscape for transplant and post-transplant care
The chapters that follow explore three themes that came to the fore and the recommendations on which there was most consensus among the experts:
Takeda therefore commissioned Charles Rivers Associates (a research consultancy firm) to undertake a landscape analysis of Canada, England, France, Germany, Italy and Spain. The assessment sought to identify commonalities and points of divergence, while considering the differences in national healthcare systems that could lead to variations in approach.
The individual country assessments were validated with national experts, to develop a long list of potential solutions to the biggest policy challenges.
Takeda then convened a multi-disciplinary and multinational expert group, bringing together
clinicians and patient representatives from the fields of solid organ and stem cell transplantation from across Europe and Canada.
Over three meetings, the expert group discussed key findings from the landscape analysis, debated which policy priorities were most common and most pressing, and suggested additional solutions for discussion. The group members welcomed the opportunity to talk with other experts with clinical and lived experience, recognising that they tend to convene with colleagues either in solid organ or in stem cell transplant and do not often come together as a holistic transplant community.
Takeda wanted to understand how the policy environment in different countries could be shaped to support national transplant services to improve patient outcomes and experience of care. We recognise that policymakers have crucial roles to play in setting national priorities, directing and encouraging investment into health and care services, and sharing best practice both within and between different countries.
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

20
Every Transplant Matters
Patient engagement and empowerment
Patients should be empowered to be an equal and active member of their treatment team. Policymakers have a key role to play in fostering patient-centric health systems and services that encourage and support patients and their caregivers to make informed decisions throughout their transplant journey.
Policy recommendations:
Ensure patients are given high quality, balanced information on the risk of post-transplant infections, as part of a comprehensive education package that recognises the complexity of patients’ lives post-transplant
Work with patient groups to translate international guidelines into patient-friendly language, so patients know what they should be expecting from their care
Audit patients’ experience of transplant care, from pre-transplant planning to follow-up care, to identify variations, challenges and best practice, and to inform strategic planning at a national and local level
Ensure patients are given high quality, balanced information on the risk of post-transplant infections, such as CMV, as part of a comprehensive education package that recognises the complexity of patients’ lives post-transplant
Management of post-transplant infections is a core part of good transplant care, and transplant teams should be routinely informing patients about the risk of different infections and how these can be managed if and when they arise. Since testing for CMV is part of pre-transplant planning, we would expect patients to be aware of CMV, why they (and their donor) would be tested for it, and how their CMV status could affect other pre-emptive treatments they might need to take.
Infections are just one of the post-transplant complications that can arise, so information on
CMV and other infections should be part of a comprehensive patient education package, that prepares and supports patients to manage different scenarios in their new life after transplant.
Patients need to take in a lot of information as they prepare for, and recover from, their transplant. High quality written information is important, so that patients can refer to it when they need to. Written information needs to be available in different languages and formats, recognising patients’ and care-givers’ differing and changing needs.
Patient organisations have a particularly important role to play in providing balanced information and guidance via their websites and patient leaflets. Clinicians can support patients and their families by ensuring that they have information about national patient advocacy organisations and local
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

21
Improving post-transplant care
Patient engagement and empowerment
Patients should be empowered to be an equal and active member of their treatment team. Policymakers have a key role to play in fostering patient-centric health systems and services that encourage and support patients and their caregivers to make informed decisions throughout their transplant journey.
Policy recommendations:
Ensure patients are given high quality, balanced information on the risk of post-transplant infections, as part of a comprehensive education package that recognises the complexity of patients’ lives post-transplant
Work with patient groups to translate international guidelines into patient-friendly language, so patients know what they should be expecting from their care
Audit patients’ experience of transplant care, from pre-transplant planning to follow-up care, to identify variations, challenges and best practice, and to inform strategic planning at a national and local level
support groups, who may be able to provide peer-to-peer advice and support. These groups can often provide valuable support for patients as they prepare for their transplant and in the weeks, months and years following.
Work with patient groups to translate international guidelines into patient-friendly language, so patients know what they should be expecting from their care
International clinical guidelines are not only important for providing peer-reviewed guidance to clinical professionals but can also be a useful benchmark against which patients can judge their care. However clinical guidelines are, by their nature, technical and do not always have an accompanying version in lay language suitable for patients and their families to understand.
Policymakers can encourage health bodies and professional societies to work with patient advocacy groups to translate clinical guidelines into patient-friendly language, so patients have an opportunity to learn what they should be expecting at each stage in their transplant journey. Patients and carers should be equipped with information to understand the expected standards of care and to have a conversation with their transplant team if they feel that their care is differing from this.
Audit patients’ experience of transplant care, from pre-transplant planning to follow-up care, to identify variations, challenges and best practice, and to inform strategic planning at a national and local level
Evidence shows that patient input into clinical guidelines development has important implications on their adoption and uptake. Patients can convey the personal impact of the disease, communicate which outcomes they find most important and identify what may be missing in care pathways. For this reason, many national and international
organisations, including the Guidelines International Network, the United States’ National Academy of Medicine,42 and the United Kingdom’s National Institute for Health and Care Excellence,43 mandate having patient input as part of clinical guideline development.
Proactively seeking feedback on patients’ experience of transplant care is critical to empowering patients and ensuring that the services in place are fit-for-purpose. The transplant team should have an open dialogue with the patient and their caregiver(s) to understand their individual goals, preferences, and needs, and to re-assess their care and support needs as part of post-transplant care management.
It is also important to aggregate transplant patients’ experiences at a bigger scale. Health systems should routinely audit and publish patients’ experience of transplant care – both in solid organ and stem cell transplants – to recognise gaps, inefficiencies and regional discrepancies and to compare them against best practices. Policymakers should be guided by these regional and national audits when assessing the resourcing and performance of transplant services to improve patient outcomes.
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

22
Every Transplant Matters
Enhancing post-transplant care delivery
Post-transplant care is a key determinant of patient outcomes and experience. Transplant recipients often face side effects, complications and difficulties during their long-term post-transplant care, affecting not only their physical but also their mental health and economic wellbeing. However, patient needs that go beyond their physical health are rarely considered.44
Policy recommendations:
Ensure transplant follow-up services are well-organised and well-funded with the multidisciplinary team capacity and capabilities needed to support holistic patient needs
Resource local testing networks to enable patients to have follow-up transplant care closer to home, including monitoring for infections
Explore and harness the potential of e-health and virtual consultations to better support transplant patients and their families closer to home
Ensure transplant follow-up services are well-organised and well-funded, with the multidisciplinary team capacity and capabilities needed to support patient needs
Multidisciplinary care is associated with better patient outcomes and lower post-transplant morbidity and mortality in both solid organ and stem cell transplant. In addition to transplant surgeons and nurses, patients benefit from having care provided by multidisciplinary teams involving a variety of specialists including infections disease specialists, physical therapists, and psychologists.47
Post-transplant care may involve years of follow up which may pose a burden on patients and carers. Patients should be involved in creating their follow-up plan with their care team, tailoring it to their individual physical, psychological and
socioeconomic needs. Policymakers need to ensure funding is made available for services to provide high quality multidisciplinary care to support patients in preparation for, and following, transplantation.
Resource local testing networks to enable patients to have follow-up transplant care closer to home, including monitoring for infections
Patients at risk of reactivated CMV infection and those receiving prophylaxis require regular monitoring for an extended period following their transplant.48 Patients may have difficulties accessing transplant centres or testing sites for many different reasons; for example they may live in a remote area or have difficulties with transport or with their mobility. Local testing networks have an important role to play in enabling transplant
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

23
Improving post-transplant care
Enhancing post-transplant care delivery
Post-transplant care is a key determinant of patient outcomes and experience. Transplant recipients often face side effects, complications and difficulties during their long-term post-transplant care, affecting not only their physical but also their mental health and economic wellbeing. However, patient needs that go beyond their physical health are rarely considered.44
Policy recommendations:
Ensure transplant follow-up services are well-organised and well-funded with the multidisciplinary team capacity and capabilities needed to support holistic patient needs
Resource local testing networks to enable patients to have follow-up transplant care closer to home, including monitoring for infections
Explore and harness the potential of e-health and virtual consultations to better support transplant patients and their families closer to home
recipients to receive testing for monitoring closer to home, with tests quickly reported back to the treatment team in charge of the patient’s care so that any emerging infection can be rapidly treated. Care teams should consider patients’ ability to access testing facilities as part of transplant care follow-up.
Policymakers should ensure that local testing networks are sufficiently resourced to support transplant patients and their families to have follow-up testing closer to home where possible, reducing the travel burden on patients and families.
Explore and harness the potential of e-health and virtual consultations to better support transplant patients and their families closer to home
The COVID-19 pandemic resulted in massive disruption to health systems worldwide. E-health and virtual consultations have emerged as important tools to provide care to vulnerable patients while minimising the risk of exposing them to COVID-19.49
E-health is commonly defined as “a type of e-government application, encompassing a wide range of devices and services that employ ICT to assist and enhance the provision of healthcare”.50 This may include anything from electronic records to apps that help patients keep track of their symptoms.
Virtual consultations, by telephone or video, have also been used by clinical teams through the pandemic to minimise extremely vulnerable patients having to travel for in-person consultations. Evidence is emerging around transplant patients’ views and experiences of virtual consultations. Some patients have welcomed the convenience of virtual appointments and found they increased their feeling of empowerment and shared decision-making, while others have found challenges around using new technologies.51,52
Health systems should examine the potential of e-health and virtual consultations to better support transplant patients and their families closer to home. For example, patients may prefer utilising e-health to keep track of their post-transplant recovery or want to swap hospital appointments for a virtual consultations in some circumstances.
Patients should have the option to discuss their preferences with their care team and explore how e-health tools can be utilised to optimise their post-transplant journey. It is important that patients are in the driving seat, and that in-person consultations remain an option for those who prefer this.
We recognise that e-health approaches are most relevant for patients who have been living with their transplant for longer and who are beyond the high risk post-transplant phase (which can persist for months or indeed years for some patients). A close link to and supervision of the transplant centre is essential for those patients who have only recently had their transplant, or who are still at high risk, and who require close monitoring with laboratory tests, clinics and medical examinations.
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

24
Case Study:
Match: A new e-platform, strategy and surveillance approach for early CMV identification and management53
Righospitalet (Copenhagen, Denmark) saw that there were variations in CMV management between its transplant departments. Donor CMV status was not routinely available to clinicians unless specifically requested. Each organ-specific transplant department was solely responsible for enforcing CMV management guidelines. There was no consistent approach to post-transplant surveillance.
The team designed a new Management of Post-transplant Infections in Collaborating Hospitals (MATCH) programme. This used pre-defined algorithms and real-time laboratory analysis to improve and standardise CMV risk assessment, identification and management across all departments.
Donors and recipients are now registered, pre-transplant, into the MATCH database. The database has a user-friendly interface with hospital laboratory databases, allowing clinicians to extract donor and recipient tests automatically in real-time.
Within a week of having their transplant, every patient having a solid organ transplant is given a personalised plan. This covers their viral risk assessment, the proposed prophylaxis strategy (according to risk and type of transplant), and a surveillance plan. All tests performed as part of surveillance are fed back to the MATCH database, and this sends alerts to clinicians in the case of any missing or concerning test results. A secondary plan is generated if an infection occurs.
Using MATCH, clinicians are now able to harvest real-time analyses, to personalise plans for individual patients and to diagnose CMV infection at an earlier stage. As a result, the team have seen fewer non-lung transplant patients presenting with CMV disease.
Every Transplant Matters
Policy recommendations:
Stimulate collaboration and research into areas of high unmet need, including supporting regional and national transplant registries and societies to collect and share evidence and data on key topics (e.g. optimal management of resistant/refractory CMV infections)
Work with national transplant societies, transplant centres, registries and patient organisations to better understand the incidence and burden of post-transplant infections, including CMV
Enable more flexible value assessment models to assess innovations that address high unmet need in small patient populations or where real-world evidence is needed, incorporating patient voices and perspectives as a direct part of the assessment
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

25
Fostering research, innovation, and data sharing
Case Study:
Match: A new e-platform, strategy and surveillance approach for early CMV identification and management53
Righospitalet (Copenhagen, Denmark) saw that there were variations in CMV management between its transplant departments. Donor CMV status was not routinely available to clinicians unless specifically requested. Each organ-specific transplant department was solely responsible for enforcing CMV management guidelines. There was no consistent approach to post-transplant surveillance.
The team designed a new Management of Post-transplant Infections in Collaborating Hospitals (MATCH) programme. This used pre-defined algorithms and real-time laboratory analysis to improve and standardise CMV risk assessment, identification and management across all departments.
Donors and recipients are now registered, pre-transplant, into the MATCH database. The database has a user-friendly interface with hospital laboratory databases, allowing clinicians to extract donor and recipient tests automatically in real-time.
Within a week of having their transplant, every patient having a solid organ transplant is given a personalised plan. This covers their viral risk assessment, the proposed prophylaxis strategy (according to risk and type of transplant), and a surveillance plan. All tests performed as part of surveillance are fed back to the MATCH database, and this sends alerts to clinicians in the case of any missing or concerning test results. A secondary plan is generated if an infection occurs.
Using MATCH, clinicians are now able to harvest real-time analyses, to personalise plans for individual patients and to diagnose CMV infection at an earlier stage. As a result, the team have seen fewer non-lung transplant patients presenting with CMV disease.
Scientific advances have transformed the outlook for many transplant patients over the past decades. Continued collaboration and innovation are essential to push the boundaries further, with a supportive policy environment both for research and development and the translation of new technologies into clinical care.
Improving post-transplant care


Dr José Luis Piñana, Hospital Clinico Universitario of Valencia, Spain

Policy recommendations:
Stimulate collaboration and research into areas of high unmet need, including supporting regional and national transplant registries and societies to collect and share evidence and data on key topics (e.g. optimal management of resistant/refractory CMV infections)
Work with national transplant societies, transplant centres, registries and patient organisations to better understand the incidence and burden of post-transplant infections, including CMV
Enable more flexible value assessment models to assess innovations that address high unmet need in small patient populations or where real-world evidence is needed, incorporating patient voices and perspectives as a direct part of the assessment
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

26
Stimulate collaboration and research into areas of high unmet need, including supporting regional, national and international registries and societies to collect and share evidence and data on key topics
Transplant registries and scientific societies at regional, national, and international levels are committed to improving patient outcomes in transplantation, through research, education, and partnership. Policymakers have a key role to play in ensuring these societies are well resourced and able to undertake this vital work, but also to ensure that public policy supports their endeavour. Increased funding to support international collaboration, coupled with harmonised regulations around research collaboration and data-sharing, will enable researchers to investigate and answer important research questions as quickly as possible.
For example, as technologies and treatments improve, the proportion of patients who may experience serious post-transplant infections is decreasing, making it harder to find and recruit patients for observational studies and clinical trials. Yet there are important research questions still to answer, for example around the management of resistant/refractory CMV in different transplant populations. This makes international collaboration essential, allowing national societies and registries across different transplant types to align on the key research questions and then to collect and pool the data and real-world evidence needed to answer them.
Patient voices are crucial in guiding research since their experiences expose where there may be unseen and unmet needs. Patient organisations should be supported to work with professional societies and national registries as they identify research questions, and to encourage patients to take part in studies and trials where appropriate. This may include comparative studies to better understand patients’ experience of care, before, during and after their transplant.
Work with national transplant societies, transplant centres, registries and patient organisations to better understand the incidence and burden of post-transplant infections, including CMV
European registries for different types of transplant (eg the European Renal Association registry for kidney replacement therapy) already collect a wealth data via national and regional registries. We would encourage these registries to review the data currently being collated on post-transplant infections and consider how this could be supplemented.
For example, it may be useful to collate additional data on patients’ experiences of post-transplant infections to compare and identify any unmet needs – physical or emotional – across different transplant types. Additional data on the incidence of refractory / resistant CMV disease would help to shape future strategies to prevent and treat disease and encourage innovation.
Collation of additional data will require investment of time and resource, including in informatics, IT infrastructure and workforce capacity. Policymakers have an important role to play in ensuring that registries at regional, national and pan-national levels have the resources they need to collate, collaborate and share data to improve our understanding and management of post-transplant care,
Every Transplant Matters
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References














Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

27
Enable more flexible value assessment models to assess innovations that address high unmet need in small patient populations or where real-world evidence is needed, incorporating patient voices and perspectives as a direct part of the assessment
Healthcare systems across Europe, Canada and other high-income countries have been looking at how to adapt value assessment models to fairly evaluate treatments that address high unmet need in small patient populations, particularly in rare diseases.54 Policymakers and regulators are also exploring ways to accelerate regulatory pathways and the health technology assessment (HTA) process, while managing uncertainties in the data.
At an EU level, the Commission has adopted a new Regulation on Health Technology Assessment, which is designed to improve patients’ access to innovative technologies.55 The Regulation will come into force in 2025 and will allow Member States’ HTA bodies to jointly horizon-scan for, and then conduct clinical assessments of, new medicines and devices. The intention is for the Regulation to support Member States in taking more evidence-based and timely decisions on patient access to new treatments and technologies, including through registries of real-world data.
CMV is not a rare disease, but refractory/resistant infections do cause serious problems for a proportion of transplant patients. As in rare diseases, it can be difficult to find and recruit sufficient patients for large-scale randomised control trials in CMV. Real world evidence from pragmatic clinical trials56 is therefore important in this small patient population.
There is increased recognition of the importance of patients’ input to HTA, alongside that of clinicians, in helping assessment bodies to weigh
and balance uncertainties.57 Patients are in a unique position to describe the impact of their condition on their quality of life, and the outcomes that matter most to them, and to challenge assumptions. By sharing their lived experience, they can help HTA bodies reach fairer and more equitable decisions.58 However, this requires patient organisations to invest time and effort in making submissions or in supporting individual patients (who may be unwell or easily fatigued) to participate in discussions; it can be particularly difficult for small organisations with limited resources to do this.
Policymakers have a key role to play in ensuring that HTA bodies recognise and value this crucial input and that patient organisations are resourced and supported to provide this where possible.
Improving post-transplant care
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References














Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

28
Case Study:
The Canadian Donation and Transplantation Research Program’s Patient, Family, and Donor Partnership Platform
The Canadian Donation and Transplantation Research Program (CDTRP) is a research network designed to increase organ and tissue donation in Canada while increasing survival and enhancing the quality of lives for Canadian’s living with a transplant.59
The CDTRP recognizes the importance of engaging patients, families and donors, ‘PFD Partners’, all aspects of its Program. By including PFD Partners, the CDTRP seeks to encourage meaningful collaboration in priority setting and conducting research that focuses on patient-identified priorities and improves patient outcomes.60
To support this, the CDTRP has created a group called the CDTRP Patient, Family, and Donor Partnership, a collaboration with patients and their families to support the work and mission of the CDTRP.61 The partnership seeks to ensure that all the committees, working groups and projects within CDTRP have meaningful patient, family and donor representation.
The Patient, Family, and Donor Partnership holds an annual Patient Family and Donor Research Forum and additional meetings with other transplant organisations. These meetings aim to empower participants through education and training opportunities, creating collaborations with clinicians and researchers across the spectrum of organ donation and transplantation research.
Patients, families and donors can find out about opportunities to participate in research programmes and surveys though a dedicated Patient Portal. The Portal also provides resources on a wide range of topics – from healthy living, to treatment, to financial assistance and loans – which are searchable by transplant type and region.
In a decade, the CDTRP has grown to include 300+ researcher members, 80 collaborators and 200+ stakeholders, all committed to establishing Canadian donation and transplantation research as the foundation to fulfil every donation opportunity and potential transplantation as a true, cost-effective cure for diseases and cancers.62
Every Transplant Matters
If you would like more information about this initiative or Takeda’s work in post-transplant care, please email Joe Brice, Head of Franchise Public Affairs & Policy, Europe & Canada

Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

29
About Takeda
Case Study:
The Canadian Donation and Transplantation Research Program’s Patient, Family, and Donor Partnership Platform
The Canadian Donation and Transplantation Research Program (CDTRP) is a research network designed to increase organ and tissue donation in Canada while increasing survival and enhancing the quality of lives for Canadian’s living with a transplant.59
The CDTRP recognizes the importance of engaging patients, families and donors, ‘PFD Partners’, all aspects of its Program. By including PFD Partners, the CDTRP seeks to encourage meaningful collaboration in priority setting and conducting research that focuses on patient-identified priorities and improves patient outcomes.60
To support this, the CDTRP has created a group called the CDTRP Patient, Family, and Donor Partnership, a collaboration with patients and their families to support the work and mission of the CDTRP.61 The partnership seeks to ensure that all the committees, working groups and projects within CDTRP have meaningful patient, family and donor representation.
The Patient, Family, and Donor Partnership holds an annual Patient Family and Donor Research Forum and additional meetings with other transplant organisations. These meetings aim to empower participants through education and training opportunities, creating collaborations with clinicians and researchers across the spectrum of organ donation and transplantation research.
Patients, families and donors can find out about opportunities to participate in research programmes and surveys though a dedicated Patient Portal. The Portal also provides resources on a wide range of topics – from healthy living, to treatment, to financial assistance and loans – which are searchable by transplant type and region.
In a decade, the CDTRP has grown to include 300+ researcher members, 80 collaborators and 200+ stakeholders, all committed to establishing Canadian donation and transplantation research as the foundation to fulfil every donation opportunity and potential transplantation as a true, cost-effective cure for diseases and cancers.62
Takeda is a patient-focused, values-based, R&D-driven global biopharmaceutical company committed to bringing better health and a brighter future to people worldwide. At Takeda, everything starts by asking: How can we do more for patients?
We are committed to discovering and delivering life-transforming treatments, guided by our commitment to patients, our people, and the planet. Takeda focuses its R&D efforts on four therapeutic areas: Oncology, Rare Diseases, Neuroscience, and Gastroenterology (GI).
We are committed to improving quality of life for patients and to working with our partners in health care in approximately 80 countries. We are keen to collaborate with partners interested in advancing transplant care, and to facilitating discussions about how the expert recommendations can be taken forward. We are considering our own role in this – at a national and international level – and where we can usefully partner to accelerate others’ efforts to improve outcomes for transplant patients.
Improving post-transplant care
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
References



22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
References


42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022
References

30
References
Every Transplant Matters
1Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe and Canada in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region / country; Region / Country: Europe / Canada; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
2European Society of Blood and Marrow Transplantation (EMBT) EMBT Activity Survey on HCT 2019. Available at: https://www.ebmt.org/registry/transplant-activity-survey
3NHS Blood and Transplant, A history of donation, transfusion, and transplantation. Available at: https://www.nhsbt.nhs.uk/who-we-are/a-history-of-donation-transfusion-and-transplantation/
4Max D Cooper, In Memoriam: Robert A Good, May 21 1922 – June 13 2003, J Immunol 2003; 171:6318-6319; Available at: https://www.jimmunol.org/content/jimmunol/171/12/6318.full.pdf
5Razonable RR, Eid AJ. Viral infections in transplant recipients, Minerva Med. 2009 Dec;100(6):479-501. Available at: https://www.minervamedica.it/en/journals/minerva-medica/article.php?cod=R10Y2009N06A0479
6Council of Europe, Human Rights Channel: Organ donation. Available at: https://www.coe.int/en/web/human-rights-channel/organ-donation
7Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
8Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
9Organ replacement in Canada: CORR annual statistics, 2020. Available at: https://www.cihi.ca/en/organ-replacement-in-canada-corr-annual-statistics-2020
10European Parliament, Organ donation and transplantation. Facts, figures, and European Union action. Available at: europarl.europa.eu/RegData/etudes/BRIE/2020/649363/EPRS_BRI(2020)649363_EN.pdf
11Global Observatory on Donation and Transplantation, Total numbers of solid organ transplants in Europe in 2020. Available at: http://www.transplant-observatory.org/data-charts-and-tables/chart/ [Search terms chosen: Question: Transplant sum: KIDNEY+HEART+LUNG+LIVER+PANCREAS+SMALL BOWEL; Geographic area: By region; Region: Europe; Type of graph: combined column and line graphs; From Year: 2020; To Year: 2020]
12CTTC National BMT Registry, 2020 Annual Report. Available at: https://cdn.ymaws.com/www.cttcanada.org/resource/resmgr/website_docs/2020-registryannualreport_v0.pdf
13Eurostat, Stem cell transplantation in the EU, October 2019. Available at: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/edn-20191011-1
14Passweg et al. Hematopoietic cell transplantation and cellular therapy survey of the EBMT: monitoring of activities and trends over 30 years (Supplementary figure 1a), Bone Marrow Transplantation (2021) 56:1651–1664. Available at https://doi.org/10.1038/s41409-021-01227-8
15Jarl J et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clinical Kidney Journal, 2018, vol. 11, no.2, 283-288.
16de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
17Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015;70(7):515–523
18Styczynski J. Who is the patient at risk of CMV recurrence: a review of the current scientific evidence with a focus on hematopoietic cell transplantation. Infect Dis Ther. 2018;7(1):1-16
19Cho S-Y, et al. Cytomegalovirus Infections after Hematopoietic Stem Cell Transplantation: Current Status and Future Immunotherapy. Int J Mol Sci. 2019;20(2666):1-17
20de la Hoz R. Diagnosis and treatment approaches to CMV infections in adult patients. J Clin Virol. 2002;25:S1-S12.
21Azevedo LS, et al. Clinics (Sao Paulo). 2015;70:515-23
22Chen J, et al. Ther Adv Hematol. 2020;11 :1-13
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents
31
Improving post-transplant care
23Fishman JA. Infection in Organ Transplantation. Am J Transplant. 2017;17:856-879.
24Kenyon M, Babic A, eds. The European Blood and Marrow Transplantation Textbook for Nurses. Springer International Publishing; 2018. doi:10.1007/978-3-319-50026-3.
25Paya CV. Economic impact of cytomegalovirus in solid organ transplantation,Transplant Infect Dis. 2001;3(suppl 3):14-19
26Robin C, et al. Economic burden of preemptive treatment of CMV infection after allogenic stem cell transplantation: a retrospective study of 208 consecutive patients, BMC Infect Dis. 2017;17:747
27Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
28Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
29Kotton CN et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2010;89:779–795.
30Kotton CN, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360.
31Kotton CN et al. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation, Transplantation 2018;102: 900-931
32Atul Humar, Patients at high risk for CMV syndrome/disease: Not only D+/R-, Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
33Hernandez et al. Cytomegalovirus transmission in mismatched solid organ transplant recipients: Are factors other than anti-viral prophylaxis at play? AmJ Transplant 2021. Available at: https://onlinelibrary.wiley.com/doi/10.1111/ajt.16734
34Nassim Kamar, CMV management practice across Europe, results of an ESOT survey. Presentation in ESOT Congress Session: Cytomegalovirus infection in organ transplantation new strategies for an old problem, 2021. Available at: https://www.youtube.com/watch?v=-DgvdrXc3ko
35Ljungman et al, Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019 Aug;19(8):e260-e272
36Boeckh M, Garrett Nichols W. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004. Mar 15;103(6): 2003-8.
37Schmidt-Hieber M et al. CMV serostatus still has an important prognostic impact in de novo acute leukaemia patients after allogenic stem cell transplantation: a report from the Acute Leukemia Working Party of EBMT. Blood. 2013 Nov 7;122(19):3359-64.
38Cantoni N et al. Evidence for a bidirectional relationship between cytomegalovirus replication and acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010 Sep;16(9):1309-14
39Shmueli E, Or R, Shapira MY, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis 2014; 209: 557–61.
40Armstrong et al. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018; 13:55
41Qaseem et al. Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–31.
42Graham et al. Clinical practice guidelines we can trust. Washington, DC: The National Academies Press; 2011.
43Jarrett L, Patient Involvement Unit (PIU). A report on a study to evaluate patient/carer membership of the first NICE Guideline Development Groups. National Institute for Clinical Excellence (NICE); 2004.
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

32
References
Every Transplant Matters
44Yang et al. The Difficulties and Needs of Organ Transplant Recipients during Postoperative Care at Home: A Systematic Review. Int J Environ Res Public Health. 2020 Aug; 17(16): 5798.
45Schmidhauser et al. The impact of multidisciplinary care on early morbidity and mortality after heart transplantation. ICVTS 2017, 25:3, 384-390
46Giaccone et al. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J Blood Med. 2020; 11: 141–162.
47Benjamin et al. Results from a multidisciplinary clinic guided by geriatric assessment before stem cell transplantation in older adults. Blood Adv 2019; 3:22, 3488–3498.
48Azervedo et al. Cytomegalovirus infection in transplant recipients. Clinics (Sao Paulo). 2015 Jul; 70(7): 515–523.
49Monaghesh et al. The role of telehealth during COVID-19 outbreak: a systematic review based on current evidence. BMC Public Health volume 20, Article number: 1193 (2020)
50Oh et al. A systematic review of published definitions. Journal of Medical Internet Research, 7 (1) (2005), p. e1
51Huuskes BM et al. Kidney transplant recipient perspectives on telehealth during the COVID-19 pandemic, Transplant International, June 2021. Available at https://doi.org/10.1111/tri.13934
52Kayser M et al. Video Consultation During the COVID-19 Pandemic. A Single Centre’s Experience with Lung Transplant Patients. Telemedicine and e-Health, Vol 27, No 7. June 2021. Available at: https://doi.org/10.1089/tmj.2020.0170
53Ekenberg C et al. For the Match Program Study Group, Evaluation of an electronic, patient-focused management system aimed at preventing cytomegalovirus disease following solid organ transplantation. Transpl Infect Dis. 2020;22:213252. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/tid.13252
54Anniemans L et al. Recommendations from the European Working Group for Value Assessment and Funding Processes in Rare Diseases (ORPH-VAL), Orphanet Journal of Rare Diseases (2017) 12:50. Available at: https://ojrd.biomedcentral.com/track/pdf/10.1186/s13023-017-0601-9.pdf
55European Commission, Health Technology Assessment: Commission welcomes the adoption of new rules to improve access to innovative technologies, December 2021. Available at: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_6771
56Roland, M. and Torgerson, D, J. ‘What are pragmatic trials’, BMJ, Vol. 316 (7127), 1998 pp. 285-285
57HTAi, Considering and Communicating Uncertainty in HTA, HTAi Global Policy Forum 2021 Background Paper. Available at: https://htai.org/wp-content/uploads/2020/12/2021-GPF-Background-Paper_Draft.pdf
58HTAi, Values and Standards for Patient Involvement in HTA, June 2014. Available at: https://transfer.htai.org/wp-content/uploads/2018/02/PCISG-Info-ValuesandStandards-30-Jun14.pdf
59The Canadian Donation and Transplantation Research Program, Our Mission, accessed February 2022
60The Canadian Donation and Transplantation Research Program, CDTRP Patient, Family and Donor Partnership Platform Terms of Reference, accessed February 2022
61The Canadian Donation and Transplantation Research Program, Patient, Family, and Donor Partnership Platform, accessed February 2022
62Patient, Family and Donor Research Forum: Post-Event Report, accessed February 2022
63The Canadian Donation and Transplantation Research Program Patient Portal, accessed February 2022
64The Canadian Donation and Transplantation Research Program, CDTRP in numbers, accessed February 2022




Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

Improving post-transplant care




Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents

Copyright 2022 Takeda Pharmaceutical Company Limited. All rights reserved. Takeda and the Takeda logo are registered trademarks of Takeda Pharmaceutical Company limited


Every Transplant Matters
Thank you for reading:
A briefing and recommendations for policymakers on improving post-transplant care
C-ANPROM/EUC/MARI/0012
Every Transplant Matters

FOREWORD
Introduction
Overview
Executive summary
Policy recommendations
Transplants: a life-saving opportunity
Post-transplant infections: the impact of CMV
Best practice in prevention and management of
CMV infections
The policy landscape for transplant and
post-transplant care
Patient engagement and empowerment
Enhancing post-transplant care delivery
Fostering research, innovation, and data sharing
About Takeda
References













Contents